at volume of 60m^3, what must the mass of water be to raise relative humidity to.40%
Humidity is the concentration of water vapour present in the air. Water vapor, the gaseous country of h2o, is by and large invisible to the human eye.[1] Humidity indicates the likelihood for atmospheric precipitation, dew, or fog to be present.
Humidity depends on the temperature and pressure of the system of interest. The same corporeality of water vapor results in higher relative humidity in absurd air than warm air. A related parameter is the dew indicate. The corporeality of h2o vapor needed to achieve saturation increases as the temperature increases. Equally the temperature of a parcel of air decreases it volition eventually reach the saturation point without adding or losing h2o mass. The amount of water vapor contained within a parcel of air can vary significantly. For example, a package of air near saturation may incorporate 28 g of water per cubic metre of air at xxx °C (86 °F), but only 8 thousand of h2o per cubic metre of air at eight °C (46 °F).
Three primary measurements of humidity are widely employed: accented, relative, and specific. Absolute humidity is expressed as either mass of water vapor per book of moist air (in grams per cubic metre)[2] or as mass of h2o vapor per mass of dry air (ordinarily in grams per kilogram).[3] Relative humidity, oft expressed as a percent, indicates a present state of absolute humidity relative to a maximum humidity given the same temperature. Specific humidity is the ratio of water vapor mass to full moist air packet mass.
Humidity plays an important function for surface life. For animal life dependent on perspiration (sweating) to regulate internal torso temperature, high humidity impairs heat exchange efficiency by reducing the rate of wet evaporation from peel surfaces. This effect tin be calculated using a heat index table, also known as a humidex.
The notion of air "belongings" h2o vapor or existence "saturated" by it is often mentioned in connection with the concept of relative humidity. This, however, is misleading—the amount of h2o vapor that enters (or tin can enter) a given infinite at a given temperature is most independent of the corporeality of air (nitrogen, oxygen, etc.) that is nowadays. Indeed, a vacuum has approximately the aforementioned equilibrium capacity to hold water vapor equally the same volume filled with air; both are given by the equilibrium vapor pressure of water at the given temperature.[four] [5] There is a very minor difference described under "Enhancement gene" below, which can exist neglected in many calculations unless high accuracy is required.
Definitions [edit]

Absolute humidity [edit]
Absolute humidity is the total mass of water vapor present in a given volume or mass of air. It does non take temperature into consideration. Absolute humidity in the temper ranges from nearly zero to roughly 30 grand (1.one oz) per cubic metre when the air is saturated at 30 °C (86 °F).[7] [8]
Absolute humidity is the mass of the water vapor , divided by the volume of the air and water vapor mixture , which tin can be expressed every bit:
The absolute humidity changes equally air temperature or pressure changes, if the volume is not stock-still. This makes information technology unsuitable for chemical applied science calculations, e.chiliad. in drying, where temperature can vary considerably. As a outcome, absolute humidity in chemical applied science may refer to mass of water vapor per unit mass of dry air, also known as the humidity ratio or mass mixing ratio (see "specific humidity" below), which is amend suited for heat and mass remainder calculations. Mass of water per unit of measurement volume every bit in the equation above is besides defined every bit volumetric humidity. Because of the potential confusion, British Standard BS 1339 [nine] suggests avoiding the term "absolute humidity". Units should e'er be carefully checked. Many humidity charts are given in g/kg or kg/kg, but any mass units may be used.
The field concerned with the written report of concrete and thermodynamic properties of gas–vapor mixtures is named psychrometrics.
Relative humidity [edit]
The relative humidity or of an air-water mixture is defined as the ratio of the partial pressure of water vapor in the mixture to the equilibrium vapor pressure of water over a flat surface of pure water[ten] at a given temperature:[11] [12] [4]
In other words, relative humidity is the ratio of how much h2o vapour is in the air and how much h2o vapour the air could potentially incorporate at a given temperature. It varies with the temperature of the air: colder air can hold less vapour. So changing the temperature of air can alter the relative humidity, even when the absolute humidity remains constant.
Chilling air increases the relative humidity, and can cause the water vapour to condense (if the relative humidity rises over 100%, the saturation signal). Likewise, warming air decreases the relative humidity. Warming some air containing a fog may cause that fog to evaporate, as the air between the water droplets becomes more able to concord h2o vapour.
Relative humidity only considers the invisible water vapour. Mists, clouds, fogs and aerosols of water do non count towards the measure out of relative humidity of the air, although their presence is an indication that a body of air may be shut to the dew point.
Relative humidity is usually expressed as a percentage; a higher percentage means that the air–water mixture is more humid. At 100% relative humidity, the air is saturated and is at its dew point. In the absence of a foreign body on which droplets or crystals tin can nucleate, the relative humidity tin can exceed 100%, in which example the air is said to be supersaturated. Introduction of some particles or a surface to a trunk of air in a higher place 100% relative humidity will allow condensation or water ice to form on those nuclei, thereby removing some of the vapour and lowering the humidity.
Relative humidity is an important metric used in atmospheric condition forecasts and reports, as information technology is an indicator of the likelihood of atmospheric precipitation, dew, or fog. In hot summertime atmospheric condition, a rise in relative humidity increases the credible temperature to humans (and other animals) past hindering the evaporation of perspiration from the skin. For example, according to the Heat Index, a relative humidity of 75% at air temperature of 80.0 °F (26.vii °C) would experience similar 83.six °F ±one.3 °F (28.7 °C ±0.7 °C).[thirteen] [xiv]
Relationship between absolute-, relative-humidity, and temperature [edit]
In the World's atmosphere at sea level:
| Temperature | Relative humidity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 10% | 20% | thirty% | 40% | l% | 60% | 70% | lxxx% | 90% | 100% | |
| 50 °C (122 °F) | 0 (0) | 8.iii (0.22) | 16.6 (0.45) | 24.9 (0.67) | 33.two (0.90) | 41.5 (1.12) | 49.8 (1.34) | 58.1 (1.57) | 66.4 (1.79) | 74.7 (2.01) | 83.0 (ii.24) |
| 45 °C (113 °F) | 0 (0) | 6.5 (0.xviii) | thirteen.1 (0.35) | 19.6 (0.53) | 26.two (0.71) | 32.7 (0.88) | 39.3 (one.06) | 45.8 (1.24) | 52.iv (1.41) | 58.nine (1.59) | 65.4 (ane.76) |
| 40 °C (104 °F) | 0 (0) | v.1 (0.xiv) | x.ii (0.28) | fifteen.3 (0.41) | 20.five (0.55) | 25.6 (0.69) | 30.7 (0.83) | 35.8 (0.97) | 40.9 (i.x) | 46.0 (1.24) | 51.1 (i.38) |
| 35 °C (95 °F) | 0 (0) | iv.0 (0.xi) | vii.ix (0.21) | 11.ix (0.32) | 15.8 (0.43) | xix.8 (0.53) | 23.8 (0.64) | 27.7 (0.75) | 31.7 (0.85) | 35.6 (0.96) | 39.6 (1.07) |
| 30 °C (86 °F) | 0 (0) | 3.0 (0.081) | vi.1 (0.16) | 9.1 (0.25) | 12.one (0.33) | 15.2 (0.41) | 18.2 (0.49) | 21.3 (0.57) | 24.three (0.66) | 27.three (0.74) | 30.4 (0.82) |
| 25 °C (77 °F) | 0 (0) | 2.three (0.062) | four.vi (0.12) | six.ix (0.19) | nine.2 (0.25) | 11.5 (0.31) | 13.8 (0.37) | 16.ane (0.43) | 18.four (0.l) | 20.7 (0.56) | 23.0 (0.62) |
| xx °C (68 °F) | 0 (0) | i.7 (0.046) | 3.5 (0.094) | five.2 (0.14) | 6.9 (0.19) | 8.7 (0.23) | 10.4 (0.28) | 12.1 (0.33) | 13.eight (0.37) | 15.6 (0.42) | 17.iii (0.47) |
| 15 °C (59 °F) | 0 (0) | 1.iii (0.035) | two.half dozen (0.070) | 3.9 (0.xi) | 5.one (0.xiv) | vi.four (0.17) | 7.7 (0.21) | 9.0 (0.24) | 10.three (0.28) | xi.5 (0.31) | 12.8 (0.35) |
| x °C (50 °F) | 0 (0) | 0.nine (0.024) | i.9 (0.051) | two.8 (0.076) | 3.8 (0.10) | 4.7 (0.xiii) | 5.half dozen (0.15) | 6.six (0.18) | vii.5 (0.xx) | 8.five (0.23) | 9.4 (0.25) |
| 5 °C (41 °F) | 0 (0) | 0.7 (0.019) | ane.4 (0.038) | 2.0 (0.054) | 2.vii (0.073) | 3.4 (0.092) | 4.1 (0.11) | 4.8 (0.xiii) | 5.4 (0.xv) | half-dozen.1 (0.sixteen) | six.8 (0.18) |
| 0 °C (32 °F) | 0 (0) | 0.5 (0.013) | 1.0 (0.027) | 1.5 (0.040) | 1.9 (0.051) | 2.4 (0.065) | 2.9 (0.078) | iii.4 (0.092) | three.ix (0.eleven) | 4.4 (0.12) | 4.8 (0.thirteen) |
| −5 °C (23 °F) | 0 (0) | 0.3 (0.0081) | 0.seven (0.019) | 1.0 (0.027) | 1.iv (0.038) | one.vii (0.046) | ii.1 (0.057) | 2.4 (0.065) | 2.seven (0.073) | 3.1 (0.084) | 3.4 (0.092) |
| −10 °C (xiv °F) | 0 (0) | 0.2 (0.0054) | 0.five (0.013) | 0.7 (0.019) | 0.ix (0.024) | 1.ii (0.032) | ane.four (0.038) | ane.6 (0.043) | 1.9 (0.051) | 2.1 (0.057) | 2.3 (0.062) |
| −fifteen °C (5 °F) | 0 (0) | 0.2 (0.0054) | 0.3 (0.0081) | 0.v (0.013) | 0.6 (0.016) | 0.eight (0.022) | 1.0 (0.027) | i.1 (0.030) | 1.3 (0.035) | 1.5 (0.040) | 1.half dozen (0.043) |
| −twenty °C (−4 °F) | 0 (0) | 0.ane (0.0027) | 0.2 (0.0054) | 0.three (0.0081) | 0.4 (0.011) | 0.4 (0.011) | 0.five (0.013) | 0.half dozen (0.016) | 0.7 (0.019) | 0.8 (0.022) | 0.nine (0.024) |
| −25 °C (−13 °F) | 0 (0) | 0.1 (0.0027) | 0.one (0.0027) | 0.2 (0.0054) | 0.2 (0.0054) | 0.3 (0.0081) | 0.3 (0.0081) | 0.iv (0.011) | 0.4 (0.011) | 0.5 (0.013) | 0.6 (0.016) |
Specific humidity [edit]
Specific humidity (or moisture content) is the ratio of the mass of water vapor to the total mass of the air package.[17] Specific humidity is approximately equal to the mixing ratio, which is defined every bit the ratio of the mass of water vapor in an air bundle to the mass of dry air for the same packet. As temperature decreases, the amount of water vapor needed to reach saturation also decreases. Equally the temperature of a parcel of air becomes lower information technology will eventually reach the indicate of saturation without adding or losing h2o mass.
[edit]
The term relative humidity is reserved for systems of water vapor in air. The term relative saturation is used to describe the coordinating property for systems consisting of a condensable phase other than water in a non-condensable phase other than air.[18]
Measurement [edit]


Hygrometer for domestic use, wet/dry psychrometer type

Thermo hygrometer displaying temperature and relative humidity
A device used to measure humidity of air is chosen a psychrometer or hygrometer. A humidistat is a humidity-triggered switch, often used to control a dehumidifier.
The humidity of an air and water vapor mixture is determined through the use of psychrometric charts if both the dry bulb temperature (T) and the wet bulb temperature (T w) of the mixture are known. These quantities are readily estimated past using a sling psychrometer.
There are several empirical formulas that can be used to estimate the equilibrium vapor pressure of water vapor equally a function of temperature. The Antoine equation is amongst the least complex of these, having only iii parameters (A, B, and C). Other formulas, such as the Goff–Gratch equation and the Magnus–Tetens approximation, are more complicated but yield improve accurateness.[ citation needed ]
The Arden Buck equation is commonly encountered in the literature regarding this topic:[19]
where is the dry-bulb temperature expressed in degrees Celsius (°C), is the accented force per unit area expressed in millibars, and is the equilibrium vapor pressure level expressed in millibars. Buck has reported that the maximal relative error is less than 0.20% between −20, and +l °C (−4, and 122 °F) when this particular form of the generalized formula is used to estimate the equilibrium vapor pressure of water.
There are various devices used to measure and regulate humidity. Calibration standards for the most accurate measurement include the gravimetric hygrometer, chilled mirror hygrometer, and electrolytic hygrometer. The gravimetric method, while the most accurate, is very cumbersome. For fast and very accurate measurement the chilled mirror method is effective.[20] For procedure on-line measurements, the nearly commonly used sensors nowadays are based on capacitance measurements to measure relative humidity,[21] frequently with internal conversions to display absolute humidity every bit well. These are cheap, simple, generally accurate and relatively robust. All humidity sensors face problems in measuring dust-laden gas, such as exhaust streams from dryers.
Humidity is likewise measured on a global scale using remotely placed satellites. These satellites are able to detect the concentration of h2o in the troposphere at altitudes between 4 and 12 km (2.5 and 7.5 mi). Satellites that can measure out water vapor have sensors that are sensitive to infrared radiation. Water vapor specifically absorbs and re-radiates radiation in this spectral band. Satellite water vapor imagery plays an of import role in monitoring climate conditions (like the formation of thunderstorms) and in the evolution of atmospheric condition forecasts.
Air density and volume [edit]
Humidity depends on water vaporization and condensation, which, in plow, mainly depends on temperature. Therefore, when applying more than pressure to a gas saturated with h2o, all components volition initially decrease in book approximately co-ordinate to the ideal gas law. Notwithstanding, some of the h2o will condense until returning to almost the same humidity as before, giving the resulting total volume deviating from what the ideal gas police force predicted. Conversely, decreasing temperature would also make some h2o condense, again making the final volume deviate from predicted by the ideal gas law. Therefore, gas volume may alternatively be expressed as the dry volume, excluding the humidity content. This fraction more accurately follows the ideal gas law. On the contrary the saturated volume is the volume a gas mixture would have if humidity was added to it until saturation (or 100% relative humidity).
Boiling air is less dense than dry air because a molecule of water (M ≈ 18 u) is less massive than either a molecule of nitrogen (G ≈ 28) or a molecule of oxygen (M ≈ 32). Nigh 78% of the molecules in dry air are nitrogen (Northward2). Another 21% of the molecules in dry air are oxygen (O2). The final 1% of dry air is a mixture of other gases.
For whatever gas, at a given temperature and force per unit area, the number of molecules nowadays in a item volume is constant – see platonic gas constabulary. So when water molecules (vapor) are introduced into that volume of dry air, the number of air molecules in the book must decrease by the same number, if the temperature and force per unit area remain constant. (The addition of water molecules, or any other molecules, to a gas, without removal of an equal number of other molecules, will necessarily require a change in temperature, pressure, or total book; that is, a alter in at least ane of these three parameters. If temperature and pressure remain constant, the book increases, and the dry air molecules that were displaced will initially movement out into the additional book, after which the mixture will eventually go uniform through diffusion.) Hence the mass per unit volume of the gas—its density—decreases. Isaac Newton discovered this phenomenon and wrote about information technology in his volume Opticks.[22]
Pressure dependence [edit]
The relative humidity of an air–water organization is dependent not only on the temperature but also on the absolute pressure level of the system of involvement. This dependence is demonstrated by because the air–water system shown below. The system is airtight (i.eastward., no matter enters or leaves the system).

If the organization at State A is isobarically heated (heating with no change in system pressure), and then the relative humidity of the system decreases because the equilibrium vapor pressure of water increases with increasing temperature. This is shown in State B.
If the system at State A is isothermally compressed (compressed with no change in system temperature), so the relative humidity of the organisation increases considering the partial pressure of water in the system increases with the volume reduction. This is shown in State C. Higher up 202.64 kPa, the RH would exceed 100% and h2o may brainstorm to condense.
If the pressure level of State A was changed past simply adding more dry air, without changing the volume, the relative humidity would not modify.
Therefore, a modify in relative humidity tin be explained by a modify in system temperature, a change in the volume of the system, or change in both of these arrangement backdrop.
Enhancement factor [edit]
The enhancement factor is defined as the ratio of the saturated vapor force per unit area of water in moist air to the saturated vapor pressure of pure water:
The enhancement factor is equal to unity for ideal gas systems. However, in real systems the interaction effects betwixt gas molecules issue in a small increase of the equilibrium vapor pressure of water in air relative to equilibrium vapor pressure level of pure water vapor. Therefore, the enhancement factor is normally slightly greater than unity for existent systems.
The enhancement gene is commonly used to right the equilibrium vapor pressure of water vapor when empirical relationships, such as those developed past Wexler, Goff, and Gratch, are used to judge the properties of psychrometric systems.
Buck has reported that, at body of water level, the vapor force per unit area of h2o in saturated moist air amounts to an increase of approximately 0.5% over the equilibrium vapor force per unit area of pure water.[nineteen]
Effects [edit]

Hygrostat gear up to 50% relative humidity

Humidor, used to control humidity of cigars
Climate control refers to the control of temperature and relative humidity in buildings, vehicles and other enclosed spaces for the purpose of providing for human condolement, health and safe, and of coming together ecology requirements of machines, sensitive materials (for case, historic) and technical processes.
Climate [edit]
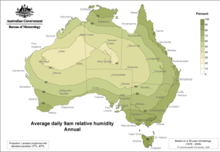
Average humidity around Australia twelvemonth-circular at nine am
lxxx–ninety%
thirty–40%
While humidity itself is a climate variable, it also affects other climate variables. Ecology humidity is affected by winds and by rainfall.
The most humid cities on earth are generally located closer to the equator, nearly coastal regions. Cities in parts of Asia and Oceania are amid the near humid. Bangkok, Ho Chi Minh Urban center, Kuala Lumpur, Hong Kong, Manila, Jakarta, Naha, Singapore, Kaohsiung and Taipei accept very loftier humidity most or all yr circular considering of their proximity to water bodies and the equator and often overcast weather condition. Some places experience extreme humidity during their rainy seasons combined with warmth giving the feel of a lukewarm sauna, such every bit Kolkata, Chennai and Cochin in India, and Lahore in Islamic republic of pakistan. Sukkur city located on the Indus River in Pakistan has some of the highest and most uncomfortable dew points in the land, frequently exceeding thirty °C (86 °F) in the Monsoon season.[23]
Loftier temperatures combine with the high dew point to create heat index in backlog of 65 °C (149 °F). Darwin experiences an extremely humid moisture season from December to Apr. Houston, Miami, San Diego, Osaka, Shanghai, Shenzhen and Tokyo as well take an extreme humid period in their summer months. During the Southward-west and North-east Monsoon seasons (respectively, late May to September and November to March), wait heavy rains and a relatively loftier humidity post-rainfall. Outside the monsoon seasons, humidity is high (in comparison to countries further from the Equator), but completely sunny days abound. In libation places such equally Northern Tasmania, Australia, loftier humidity is experienced all year due to the ocean between mainland Commonwealth of australia and Tasmania. In the summer the hot dry air is absorbed by this bounding main and the temperature rarely climbs in a higher place 35 °C (95 °F).
Global climate [edit]
Humidity affects the energy budget and thereby influences temperatures in two major ways. First, water vapor in the atmosphere contains "latent" energy. During transpiration or evaporation, this latent estrus is removed from surface liquid, cooling the world'due south surface. This is the biggest non-radiative cooling effect at the surface. Information technology compensates for roughly 70% of the average internet radiative warming at the surface.
2d, water vapor is the near abundant of all greenhouse gases. H2o vapor, like a green lens that allows green calorie-free to pass through information technology but absorbs red light, is a "selective absorber". Like the other greenhouse gasses, water vapor is transparent to most solar energy. However, information technology absorbs the infrared energy emitted (radiated) upwards past the earth's surface, which is the reason that humid areas feel very little nocturnal cooling but dry out desert regions cool considerably at nighttime. This selective absorption causes the greenhouse upshot. Information technology raises the surface temperature substantially above its theoretical radiative equilibrium temperature with the lord's day, and water vapor is the cause of more than of this warming than any other greenhouse gas.
Unlike nigh other greenhouse gases, still, h2o is not merely below its boiling point in all regions of the World, but below its freezing point at many altitudes. Equally a condensible greenhouse gas, it precipitates, with a much lower scale pinnacle and shorter atmospheric lifetime — weeks instead of decades. Without other greenhouse gases, Earth's blackbody temperature, below the freezing point of h2o, would cause water vapor to be removed from the atmosphere.[24] [25] [26] H2o vapor is thus a "slave" to the non-condensible greenhouse gases.[27] [28] [29]
Beast and plant life [edit]

Tillandsia usneoides in Tropical firm, Majestic Botanic Gardens, Kew. It is growing where the climate is warm enough and has a relatively loftier average humidity.
Humidity is one of the fundamental abiotic factors that defines whatever habitat (the tundra, wetlands, and the desert are a few examples), and is a determinant of which animals and plants can thrive in a given environment.[30]
The human torso dissipates heat through perspiration and its evaporation. Rut convection, to the surrounding air, and thermal radiation are the primary modes of heat ship from the body. Under conditions of high humidity, the charge per unit of evaporation of sweat from the skin decreases. Also, if the atmosphere is as warm as or warmer than the skin during times of high humidity, blood brought to the trunk surface cannot dissipate heat by conduction to the air. With so much blood going to the external surface of the trunk, less goes to the agile muscles, the brain, and other internal organs. Physical strength declines, and fatigue occurs sooner than it would otherwise. Alertness and mental chapters also may be affected, resulting in oestrus stroke or hyperthermia.
Human being comfort [edit]
Although humidity is an important factor for thermal comfort, humans are more sensitive to variations in temperature than they are to changes in relative humidity.[31] Humidity has a small issue on thermal comfort outdoors when air temperatures are low, a slightly more pronounced effect at moderate air temperatures, and a much stronger influence at higher air temperatures.[32]
Humans are sensitive to boiling air because the homo body uses evaporative cooling every bit the chief mechanism to regulate temperature. Under boiling conditions, the rate at which perspiration evaporates on the peel is lower than information technology would be nether arid conditions. Because humans perceive the rate of heat transfer from the body rather than temperature itself, we feel warmer when the relative humidity is high than when information technology is depression.
Humans can be comfortable inside a broad range of humidities depending on the temperature—from 30 to seventy%[33]—but ideally not higher up the Absolute (60°F Dew Bespeak),[34] between 40%[35] and threescore%.[36] In full general, higher temperatures will crave lower humidities to accomplish thermal comfort compared to lower temperatures, with all other factors held abiding. For case, with article of clothing level = 1, metabolic rate = 1.1, and air speed 0.1 yard/s, a alter in air temperature and mean radiant temperature from xx °C to 24 °C would lower the maximum acceptable relative humidity from 100% to 65% to maintain thermal comfort conditions. The CBE Thermal Comfort Tool can be used to demonstrate the effect of relative humidity for specific thermal comfort conditions and it tin be used to demonstrate compliance with ASHRAE Standard 55-2017.[37]
Some people experience difficulty animate in boiling environments. Some cases may perhaps be related to respiratory weather such as asthma, while others may exist the product of feet. Sufferers will ofttimes hyperventilate in response, causing sensations of numbness, faintness, and loss of concentration, amidst others.[38]
Very low humidity can create discomfort, respiratory problems, and aggravate allergies in some individuals. Depression humidity causes tissue lining nasal passages to dry out, crack and go more susceptible to penetration of rhinovirus cold viruses.[39] Extremely low (below twenty%) relative humidities may as well cause centre irritation.[forty] [41] The use of a humidifier in homes, especially bedrooms, can help with these symptoms.[42] Indoor relative humidities should be kept above 30% to reduce the likelihood of the occupant'due south nasal passages drying out, especially in winter.[40] [43] [44]
Air-conditioning reduces discomfort by reducing not simply temperature just humidity besides. Heating cold outdoor air can decrease relative humidity levels indoors to below 30%.[45] According to ASHRAE Standard 55-2017: Thermal Environmental Weather condition for Human Occupancy, indoor thermal condolement can be achieved through the PMV method with relative humidities ranging from 0% to 100%, depending on the levels of the other factors contributing to thermal condolement.[46] However, the recommended range of indoor relative humidity in air conditioned buildings is generally 30–60%.[47] [48]
Human health [edit]
Higher humidity reduces the infectivity of aerosolized influenza virus. A study concluded, "Maintaining indoor relative humidity >forty% volition significantly reduce the infectivity of aerosolized virus."[49]
Mucociliary clearance in the respiratory tract is also hindered by low humidity. One study in dogs found that mucus transport was lower at an accented humidity of nine m water/m3 than at 30 m water/mthree.[50]
Increased humidity can also lead to changes in total body water that usually leads to moderate weight gain, especially if one is acclimated to working or exercising in hot and humid atmospheric condition.[51]
Building structure [edit]

Common construction methods ofttimes produce edifice enclosures with a poor thermal purlieus, requiring an insulation and air bulwark system designed to retain indoor environmental conditions while resisting external environmental conditions.[52] The energy-efficient, heavily sealed compages introduced in the 20th century likewise sealed off the motion of moisture, and this has resulted in a secondary problem of condensation forming in and around walls, which encourages the evolution of mold and mildew. Additionally, buildings with foundations non properly sealed will allow water to flow through the walls due to capillary action of pores found in masonry products. Solutions for energy-efficient buildings that avoid condensation are a current topic of architecture.
For climate control in buildings using HVAC systems, the key is to maintain the relative humidity at a comfortable range—low enough to exist comfortable but high enough to avoid problems associated with very dry air.
When the temperature is high and the relative humidity is depression, evaporation of water is rapid; soil dries, moisture clothes hung on a line or rack dry quickly, and perspiration readily evaporates from the skin. Wooden furniture tin can compress, causing the paint that covers these surfaces to fracture.
When the temperature is low and the relative humidity is loftier, evaporation of water is slow. When relative humidity approaches 100%, condensation can occur on surfaces, leading to problems with mold, corrosion, disuse, and other moisture-related deterioration. Condensation tin can pose a safety gamble as information technology can promote the growth of mold and woods rot every bit well as possibly freezing emergency exits shut.
Certain production and technical processes and treatments in factories, laboratories, hospitals, and other facilities require specific relative humidity levels to exist maintained using humidifiers, dehumidifiers and associated control systems.
Vehicles [edit]
The basic principles for buildings, in a higher place, also apply to vehicles. In addition, there may be condom considerations. For example, high humidity inside a vehicle can lead to problems of condensation, such as misting of windshields and shorting of electrical components. In vehicles and pressure vessels such as pressurized airliners, submersibles and spacecraft, these considerations may be critical to safety, and circuitous environmental command systems including equipment to maintain pressure level are needed.
Aviation [edit]
Airliners operate with low internal relative humidity, often under 20%,[53] especially on long flights. The depression humidity is a upshot of drawing in the very cold air with a depression absolute humidity, which is found at airliner cruising altitudes. Subsequent warming of this air lowers its relative humidity. This causes discomfort such as sore eyes, dry out skin, and drying out of mucosa, but humidifiers are not employed to heighten it to comfortable mid-range levels because the volume of water required to be carried on board can be a significant weight penalty. As airliners descend from colder altitudes into warmer air (perhaps even flying through clouds a few k feet above the footing), the ambience relative humidity can increment dramatically. Some of this moist air is usually fatigued into the pressurized aircraft cabin and into other non-pressurized areas of the aircraft and condenses on the cold aircraft pare. Liquid water can usually be seen running along the aircraft skin, both on the inside and outside of the motel. Because of the desperate changes in relative humidity within the vehicle, components must be qualified to operate in those environments. The recommended environmental qualifications for most commercial aircraft components is listed in RTCA DO-160.
Cold, humid air can promote the formation of ice, which is a danger to aircraft as it affects the fly profile and increases weight. Carburetor engines take a further danger of ice forming inside the carburetor. Aviation weather reports (METARs) therefore include an indication of relative humidity, usually in the grade of the dew point.
Pilots must take humidity into account when calculating takeoff distances, because high humidity requires longer runways and will decrease climb performance.
Density altitude is the altitude relative to the standard atmosphere atmospheric condition (International Standard Temper) at which the air density would exist equal to the indicated air density at the place of observation, or, in other words, the acme when measured in terms of the density of the air rather than the altitude from the basis. "Density Altitude" is the pressure altitude adjusted for non-standard temperature.
An increment in temperature, and, to a much lesser degree, humidity, will crusade an increase in density distance. Thus, in hot and humid conditions, the density altitude at a particular location may be significantly higher than the true altitude.
Electronics [edit]

Desiccant bag (silica gel), ordinarily included in packages containing electronic products to command humidity
Electronic devices are frequently rated to operate just nether certain humidity conditions (eastward.thou., 10% to ninety%). At the top cease of the range, moisture may increment the conductivity of permeable insulators leading to malfunction. Too depression humidity may make materials breakable. A particular danger to electronic items, regardless of the stated operating humidity range, is condensation. When an electronic particular is moved from a common cold place (e.k., garage, car, shed, air conditioned space in the tropics) to a warm humid identify (business firm, outside tropics), condensation may coat circuit boards and other insulators, leading to brusk circuit inside the equipment. Such short circuits may cause substantial permanent harm if the equipment is powered on earlier the condensation has evaporated. A like condensation effect can ofttimes be observed when a person wearing spectacles comes in from the cold (i.due east. the glasses become foggy).[54] Information technology is appropriate to allow electronic equipment to acclimatise for several hours, after being brought in from the common cold, before powering on. Some electronic devices tin detect such a change and indicate, when plugged in and usually with a pocket-sized droplet symbol, that they cannot be used until the risk from condensation has passed. In situations where time is disquisitional, increasing air menstruum through the device'southward internals, such as removing the side panel from a PC case and directing a fan to blow into the case, will reduce significantly the time needed to acclimatise to the new environment.
In contrast, a very depression humidity level favors the build-up of static electricity, which may result in spontaneous shutdown of computers when discharges occur. Apart from spurious erratic function, electrostatic discharges can crusade dielectric breakdown in solid land devices, resulting in irreversible damage. Information centers often monitor relative humidity levels for these reasons.
Industry [edit]
Loftier humidity can frequently have a negative effect on the capacity of chemical plants and refineries that use furnaces as part of a certain processes (e.one thousand., steam reforming, wet sulfuric acid processes). For example, because humidity reduces ambient oxygen concentrations (dry air is typically twenty.nine% oxygen, but at 100% relative humidity the air is twenty.4% oxygen), flue gas fans must intake air at a higher rate than would otherwise exist required to maintain the same firing rate.[55]
Baking [edit]
High humidity in the oven, represented past an elevated wet-bulb temperature, increases the thermal conductivity of the air around the baked particular, leading to a quicker blistering procedure or even burning. Conversely, low humidity slows the baking procedure downwardly.[56]
Other important facts [edit]

At 100% relative humidity, air is saturated and at its dew point: the h2o vapor force per unit area would let neither evaporation of nearby liquid water nor condensation to grow the nearby water; neither sublimation of nearby water ice nor deposition to abound the nearby ice.
Relative humidity can exceed 100%, in which case the air is supersaturated. Cloud formation requires supersaturated air. Cloud condensation nuclei lower the level of supersaturation required to form fogs and clouds - in the absence of nuclei around which droplets or ice tin form, a higher level of supersaturation is required for these droplets or water ice crystals to class spontaneously. In the Wilson deject sleeping room, which is used in nuclear physics experiments, a state of supersaturation is created within the sleeping accommodation, and moving subatomic particles act equally condensation nuclei so trails of fog bear witness the paths of those particles.
For a given dew betoken and its corresponding absolute humidity, the relative humidity will modify inversely, albeit nonlinearly, with the temperature. This is because the partial pressure of water increases with temperature—the operative principle behind everything from pilus dryers to dehumidifiers.
Due to the increasing potential for a college water vapor partial pressure at higher air temperatures, the h2o content of air at sea level can get as loftier every bit 3% by mass at 30 °C (86 °F) compared to no more than about 0.5% by mass at 0 °C (32 °F). This explains the depression levels (in the absence of measures to add together moisture) of humidity in heated structures during winter, resulting in dry peel, itchy eyes, and persistence of static electric charges. Even with saturation (100% relative humidity) outdoors, heating of infiltrated outside air that comes indoors raises its moisture chapters, which lowers relative humidity and increases evaporation rates from moist surfaces indoors (including human bodies and household plants.)
Similarly, during summer in boiling climates a great deal of liquid water condenses from air cooled in air conditioners. Warmer air is cooled beneath its dew point, and the excess h2o vapor condenses. This phenomenon is the same as that which causes water aerosol to form on the exterior of a cup containing an ice-cold drink.
A useful rule of thumb is that the maximum absolute humidity doubles for every 20 °F (11 °C) increase in temperature. Thus, the relative humidity volition drop past a gene of 2 for each xx °F (11 °C) increase in temperature, bold conservation of absolute moisture. For example, in the range of normal temperatures, air at 68 °F (twenty °C) and 50% relative humidity will become saturated if cooled to 50 °F (10 °C), its dew indicate, and 41 °F (v °C) air at lxxx% relative humidity warmed to 68 °F (twenty °C) volition have a relative humidity of only 29% and feel dry. By comparison, thermal comfort standard ASHRAE 55 requires systems designed to control humidity to maintain a dew indicate of 16.viii °C (62.ii °F) though no lower humidity limit is established.[46]
Water vapor is a lighter gas than other gaseous components of air at the same temperature, so boiling air volition tend to rise by natural convection. This is a mechanism behind thunderstorms and other weather phenomena. Relative humidity is often mentioned in atmospheric condition forecasts and reports, every bit it is an indicator of the likelihood of dew, or fog. In hot summer weather, it besides increases the apparent temperature to humans (and other animals) by hindering the evaporation of perspiration from the skin as the relative humidity rises. This outcome is calculated as the heat alphabetize or humidex.
A device used to measure humidity is chosen a hygrometer; one used to regulate it is called a humidistat, or sometimes hygrostat. (These are analogous to a thermometer and thermostat for temperature, respectively.)
Encounter also [edit]
- Concentration
- Dew bespeak depression
- Rut index
- Humidity buffering
- Humidity indicator carte
- Humidity indicator
- Psychrometrics
- Saturation vapor density
- Water activity
References [edit]
Citations [edit]
- ^ "What is Water Vapor". Retrieved 2012-08-28 .
- ^ Wyer, Samuel S. (1906). "Primal Physical Laws and Definitions". A Treatise on Producer-Gas and Gas-Producers. McGraw-Colina Book Company. p. 23.
- ^ Perry, R.H. and Green, D.Due west, (2007) Perry'south Chemical Engineers' Handbook (8th Edition), Section 12, Psychrometry, Evaporative Cooling and Solids Drying McGraw-Colina, ISBN 978-0-07-151135-iii
- ^ a b "Water Vapor Myths: A Brief Tutorial". www.atmos.umd.edu. Archived from the original on 2016-01-25.
- ^ Fraser, Alistair B. "Bad Clouds FAQ". www.ems.psu.edu. Archived from the original on 2006-06-17.
- ^ "Antarctic Air Visits Paranal". ESO Picture show of the Week . Retrieved four February 2014.
- ^ "Climate - Humidity indexes". Encyclopaedia Britannica . Retrieved 15 February 2018.
- ^ "Climate/humidity table". Transport Information Service of the German language Insurance Association . Retrieved fifteen Feb 2018.
- ^ British Standard BS 1339 (revised), Humidity and Dewpoint, Parts 1-3 (2002-2007)
- ^ "Water Vapor Myths: A Brief Tutorial".
- ^ Perry, R.H. and Green, D.West, Perry's Chemic Engineers' Handbook (7th Edition), McGraw-Hill, ISBN 0-07-049841-5, Eqn 12-7
- ^ Lide, David (2005). CRC Handbook of Chemistry and Physics (85 ed.). CRC Printing. pp. 15–25. ISBN0-8493-0485-7.
- ^ Lans P. Rothfusz. "The Rut Index 'Equation' (or, More Than You Always Wanted to Know Nigh Heat Index)", Scientific Services Segmentation (NWS Southern Region Headquarters), 1 July 1990 "Archived re-create" (PDF). Archived from the original (PDF) on 2011-12-01. Retrieved 2011-07-23 .
{{cite spider web}}: CS1 maint: archived re-create as championship (link) - ^ Steadman, R. G. (1979). "The Assessment of Sultriness. Part I: A Temperature-Humidity Alphabetize Based on Homo Physiology and Clothing Scientific discipline". Journal of Practical Meteorology. xviii (seven): 861–873. Bibcode:1979JApMe..eighteen..861S. doi:10.1175/1520-0450(1979)018<0861:TAOSPI>two.0.CO;ii. ISSN 0021-8952.
- ^ "Climate/humidity table – Transport Informations Service". www.tis-gdv.de . Retrieved 2021-06-17 .
- ^ "Absolute Humidity Table" (PDF). mercury.pr.erau.edu . Retrieved 2021-06-17 .
{{cite web}}: CS1 maint: url-condition (link) - ^ Seidel, Dian. "What is atmospheric humidity and how is it measured? (broken link)". National Oceanic and Atmospheric Administration. National Oceanic and Atmospheric Assistants. Archived from the original on 18 October 2017. Retrieved 3 March 2017.
- ^ "Vapor-Liquid/Solid System, 201 Grade Page". University of Arizona. Archived from the original on May 8, 2006.
- ^ a b Buck 1981, pp. 1527–1532.
- ^ Pieter R. Wiederhold. 1997. Water Vapor Measurement, Methods and Instrumentation. Marcel Dekker, New York, NY ISBN 9780824793197
- ^ "BS1339" Part 3
- ^ Isaac Newton (1704). Opticks. Dover. ISBN978-0-486-60205-9.
- ^ "Weather History for Sukkur, Pakistan – Weather Hush-hush".
- ^ "Blackbody Radiations".
- ^ "Lecture notes". Archived from the original on 2017-10-23. Retrieved 2015-01-11 .
- ^ "Radiative Balance, Earth's Temperature, and Greenhouse Gases (lecture notes)".
- ^ Alley, R. (2014). "GEOSC ten Optional Enrichment Article 1". Archived from the original on 2018-09-08. Retrieved 2015-01-11 .
- ^ Businger, S. "Lecture 28: Futurity Global Warming Modeling Climate Alter" (PDF). Archived from the original (PDF) on 2015-01-xxx.
- ^ Schwieterman, Due east. "Comparing the Greenhouse Effect on World, Mars, Venus, and Titan: Present Day and through Time" (PDF).
- ^ C.Michael Hogan. 2010. Abiotic factor. Encyclopedia of Earth. eds Emily Monosson and C. Cleveland. National Council for Science and the Environment Archived June eight, 2013, at the Wayback Car. Washington DC
- ^ Fanger 1970, p. 48.
- ^ Bröde et al. 2011, pp. 481–494.
- ^ Gilmore 1972, p. 99.
- ^ [i] ASHRAE Std 62.1-2019
- ^ "Winter Indoor Comfort and Relative Humidity", Information please (database), Pearson, 2007, archived from the original on 2013-04-27, retrieved 2013-05-01 ,
…past increasing the relative humidity to above l% within the higher up temperature range, 80% or more than of all average dressed persons would feel comfortable.
- ^ "Recommended relative humidity level", The engineering toolbox, archived from the original on 2013-05-11, retrieved 2013-05-01 ,
Relative humidity higher up threescore% feels uncomfortable wet. Homo comfort requires the relative humidity to be in the range 25–threescore% RH.
- ^ Schiavon, Hoyt & Piccioli 2013, pp. 321–334.
- ^ "Rut and humidity - the lung association". www.lung.ca. 26 Baronial 2014. Retrieved 14 March 2018.
- ^ "What causes the common cold?". University of Rochester Medical Eye . Retrieved 2016-01-24 .
- ^ a b Arundel et al. 1986, pp. 351–361.
- ^ "Indoor air quality testing". Archived from the original on 2017-09-21.
- ^ "Nosebleeds". WebMD Medical Reference . Retrieved 2015-11-01 .
- ^ "Indoor Air Quality" (PDF). NH DHHS, Division of Public Health Services. Archived (PDF) from the original on 2015-09-22. Retrieved 2016-01-24 .
- ^ "Schoolhouse Indoor Air Quality: All-time Direction Practices Transmission" (PDF). Washington Country Department of Health. Nov 2003. Retrieved 2015-11-01 .
- ^ "Optimum Humidity Levels for Home". AirBetter.org. iii August 2014.
- ^ a b ASHRAE Standard 55 (2017). "Thermal Environmental Conditions for Human being Occupancy".
- ^ Wolkoff & Kjaergaard 2007, pp. 850–857.
- ^ ASHRAE Standard 160 (2016). "Criteria for Moisture-Command Pattern Analysis in Buildings"
- ^ Noti, John D.; Blachere, Francoise M.; McMillen, Cynthia G.; Lindsley, William G.; Kashon, Michael L.; Slaughter, Denzil R.; Beezhold, Donald H. (2013). "High Humidity Leads to Loss of Infectious Influenza Virus from Simulated Coughs". PLOS Ane. 8 (two): e57485. Bibcode:2013PLoSO...857485N. doi:x.1371/periodical.pone.0057485. PMC3583861. PMID 23460865.
- ^ Pieterse, A; Hanekom, SD (2018). "Criteria for enhancing fungus transport: a systematic scoping review". Multidisciplinary Respiratory Medicine. 13: 22. doi:ten.1186/s40248-018-0127-6. PMC6034335. PMID 29988934.
- ^ "To what degree is a person's trunk weight affected by the ambient temperature and humidity? Do we conserve or release water as the climate changes?". Scientific American . Retrieved 2021-06-09 .
- ^ "Free publications". Archived from the original on 2014-10-03. Retrieved 2013-12-23 .
- ^ "Airplane Humidity". Aviator Atlas. 5 April 2020. Retrieved 11 September 2020.
- ^ "Fogging Glasses". Archived from the original on 2015-02-26. Retrieved 2012-08-08 .
- ^ "Everything You Demand to Know About Combustion Chemistry & Analysis – Industrial Controls".
- ^ "Why is humidity of import in cooking?".
General sources [edit]
- Arundel, A. Five.; Sterling, Due east. Thou.; Biggin, J. H.; Sterling, T. D. (1986). "Indirect health effects of relative humidity in indoor environments". Environ. Health Perspect. 65: 351–61. doi:10.1289/ehp.8665351. PMC1474709. PMID 3709462.
- Bröde, Peter; Fiala, Dusan; Błażejczyk, Krzysztof; Holmér, Ingvar; Jendritzky, Gerd; Kampmann, Bernhard; Tinz, Birger; Havenith, George (2011-05-31). "Deriving the operational procedure for the Universal Thermal Climate Index (UTCI)". International Journal of Biometeorology. 56 (three): 481–494. doi:10.1007/s00484-011-0454-1. ISSN 0020-7128. PMID 21626294. S2CID 37771005.
- Buck, Arden 50. (1981). "New Equations for Computing Vapor Pressure level and Enhancement Cistron". Journal of Applied Meteorology. twenty (12): 1527–1532. Bibcode:1981JApMe..20.1527B. doi:x.1175/1520-0450(1981)020<1527:NEFCVP>ii.0.CO;2. ISSN 0021-8952.
- Fanger, P. O. (1970). Thermal Comfort: Analysis and Applications in Environmental Applied science. Danish Technical Press. ISBN978-87-571-0341-0.
- Gilmore, C. P. (September 1972). "More than Condolement for Your Heating Dollar". Popular Science. p. 99.
- Himmelblau, David One thousand. (1989). Basic Principles And Calculations In Chemic Technology. Prentice Hall. ISBN0-xiii-066572-10.
- Lide, David (2005). CRC Handbook of Chemistry and Physics (85 ed.). CRC Press. ISBN9780849304859.
- Perry, R.H.; Green, D.West (1997). Perry's Chemical Engineers' Handbook (seventh ed.). McGraw-Hill. ISBN0-07-049841-5.
- Schiavon, Stefano; Hoyt, Tyler; Piccioli, Alberto (2013-12-27). "Spider web application for thermal comfort visualization and adding co-ordinate to ASHRAE Standard 55". Building Simulation. 7 (4): 321–334. doi:10.1007/s12273-013-0162-three. ISSN 1996-3599. S2CID 56274353.
- Wolkoff, Peder; Kjaergaard, Søren Yard. (Baronial 2007). "The dichotomy of relative humidity on indoor air quality". Environment International. 33 (6): 850–857. doi:10.1016/j.envint.2007.04.004. ISSN 0160-4120. PMID 17499853.
- U.s. Environmental Protection Agency, "IAQ in Big Buildings". Retrieved Jan. nine, 2006.
External links [edit]
| | Wait up humidity in Wiktionary, the free dictionary. |
- Current map of global relative humidity
Source: https://en.wikipedia.org/wiki/Humidity















0 Response to "at volume of 60m^3, what must the mass of water be to raise relative humidity to.40%"
Post a Comment